
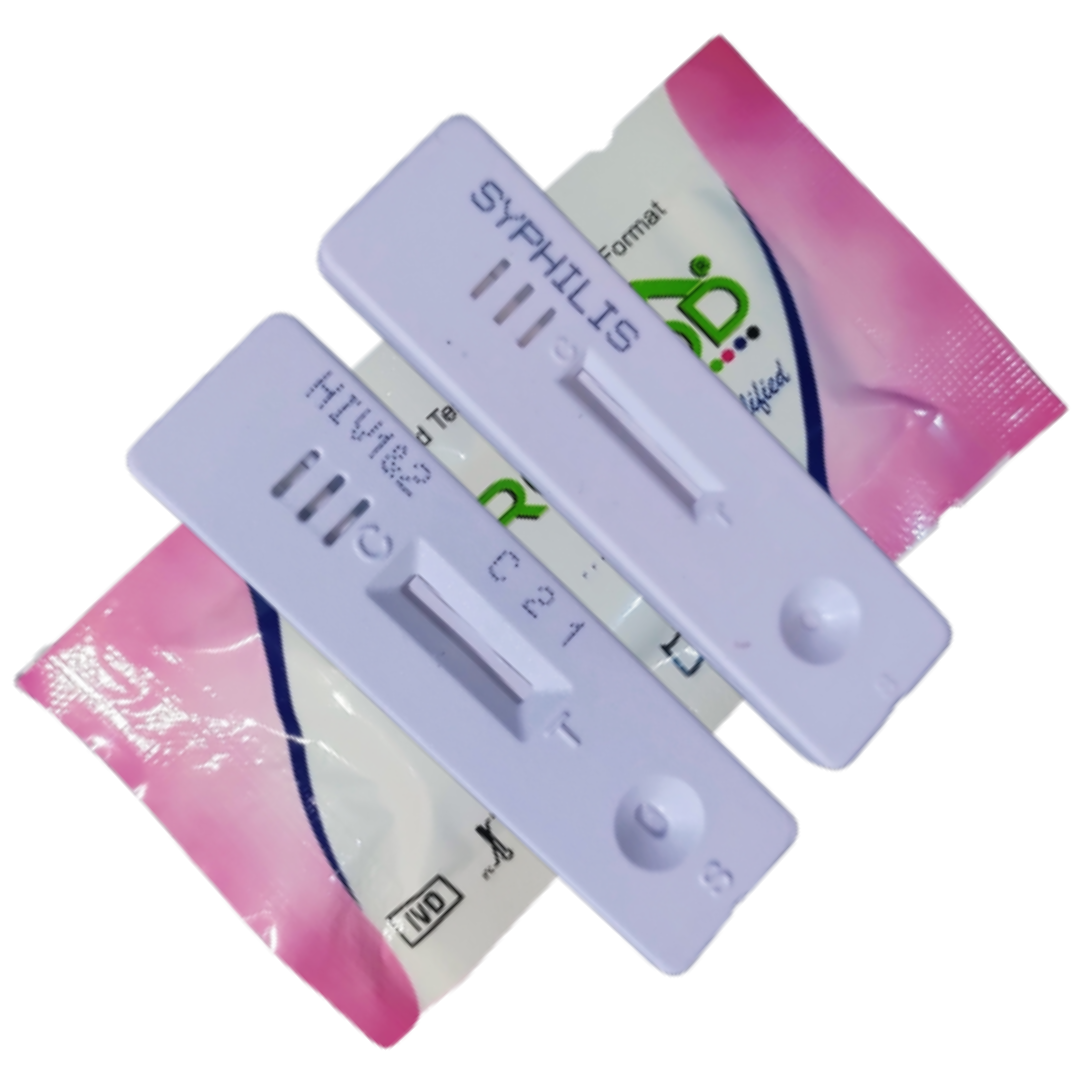
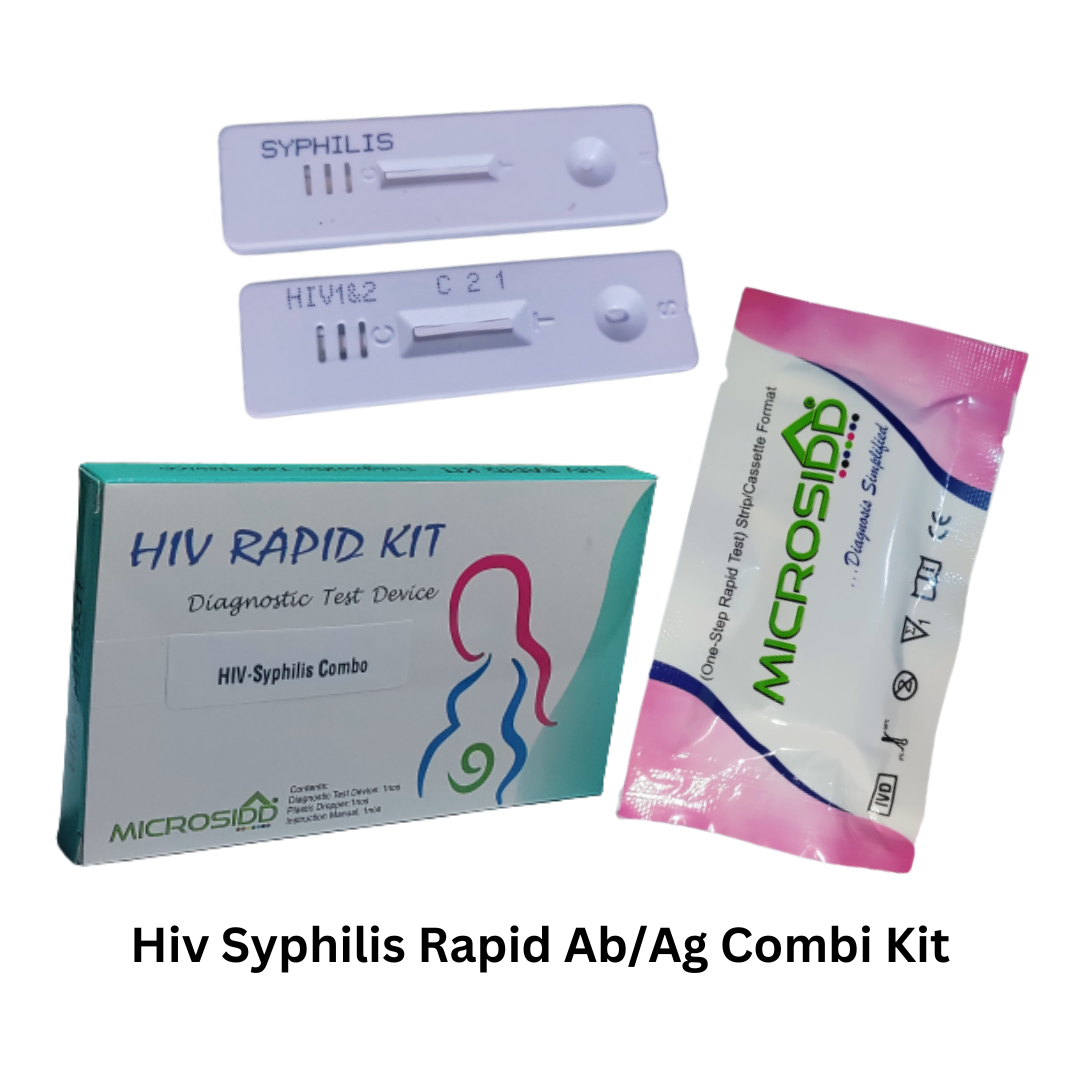

HIV-Syphilis Combi Kit
Description
HIV-Syphilis Combi Kit
1 HIV-Syphilis Test Kit trial pack
Microsidd brings Best in class products with innovation, now do hiv and syphilis test at home with ease and detect Antibodies and antigens if present in sample, test anytime after 30 days till lifetime, results are 99.80% accurate if done as per instructions provided, just need two drops of sample on each cassette and two drops buffer provided. do not consider any band appears after 20 minutes usually happens due to overflow of the sample. only first reaction upto 15 minutes is considered as final result.
How to test hiv in hindi Watch video https://youtu.be/vUxMCo1WgIo
How to test hiv in english Watch video https://youtu.be/zKK7j3kB4cw
Each kit contains:
Individually sealed foil pouch containing:
One HIV-Syphilis cassette device
One desiccant
Specimen transfer device
Sample diluent( buffer )
One package insert (instruction for use)
Note: use proper gloves while testing others for self test no problem, touching any accessory dont harm, dispose the kit after use properly.
watch these videos and then test: https://youtu.be/LWJf61qU55U?si=TG5kX0wwyxXqQ8iR
https://youtu.be/zKK7j3kB4cw?si=_lv9xIHkUALlqMER
Following an exposure that leads to HIV-Syphilis infection, the amount of time during which no existing diagnostic test is capable of detecting HIV is called the eclipse period.2
The time between potential HIV exposure and an accurate test result is referred to as the window period. Improvements in testing technology continue to reduce the detection window period, and, therefore, the time to diagnosis and treatment of early HIV infection. As seen in the figure, each type of HIV test has its own testing window, with the NAT capable of detecting HIV the earliest, followed by the antigen/antibody combination test, and lastly, the antibody test.
HIV Program Evaluations :
https://www.cdc.gov/hiv/pdf/library/reports/cdc-hiv-annual-HIV-testing-report-2020.pdf
https://www.cdc.gov/hiv/pdf/research/evaluation_HIVTesting_BudgetAllocation.pdf
https://www.cdc.gov/hiv/pdf/testing_resources_CDC_Evaluation_Toolkit_Routine_HIV_Screening.pdf
Legal Disclaimer: this is an diagnostic product to be used in or by medical professional expert supervision only Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and cross verify with ELISA / PCR Methods at your diagnostic centre or referral diagnostic laboratory
People also purchased
https://microsidd.com/en/4th-generation-hiv-test-kit/
https://microsidd.com/en/4th-generation-kit-hiv-hcv/
hiv testing kit
hiv rapid test kit
hiv test kit online
hiv test kit
hiv test kit price

